
Control over such multi-compartment levels would allow more precise encapsulation and the development of more advanced materials. Microfluidic devices have been designed to produce such systems, but controlling the number, size and ratio of droplets at each level is difficult, especially when developing a system that has different types of emulsion droplets at the same level. Multiple emulsions are liquid systems in which emulsion droplets are placed inside each other, each droplet smaller than the last, creating ’levels’. This system could be used to encapsulate incompatible drug ingredients and to design multi-compartment materials, they say. Many ethanol plants use chemistry like Ascent, which was developed by Trucent and Croda with existing Croda patented and bio-based ingredients, to extract more DCO for less money .Scientists in China have developed a device that can control the production of multiple emulsion systems. But the enzyme technology used to increase oil extraction from ethanol stillage tends to trap a good deal of oil in solution, creating extremely “tightly bound” emulsions that resist mechanical separation unless emulsion breakers are used. DCO (distillers corn oil) has become a vital co-product for most ethanol plants. But they are especially important in certain industries, like ethanol. These larger droplets are much easier to separate and capture via centrifugation.Įmulsion breakers generally make it easier/less energy intensive to separate fluids. The oil droplets can then begin to flocculate and coalesce, forming ever-larger droplets and globules (as illustrated from the bottom up on the middle of the diagram). When the chemistry penetrates the emulsion, it disrupts any hydrophilic/hydrophobic layers protecting these dispersed droplets. Before dosing, the oil droplets in your fluid are quite scattered (as seen on the far left of the figure above). It breaks the emulsion up by separating water and oil components.

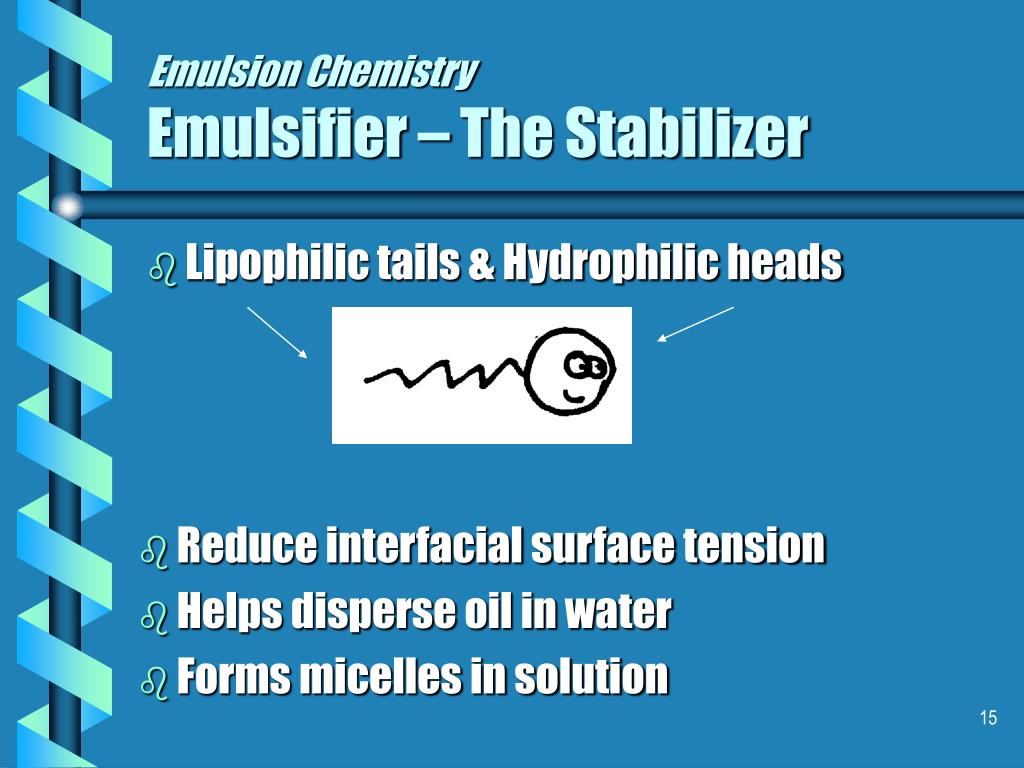
The emulsion breaker behaves exactly the opposite of the emulsifier (hence the name “demulsifier”). This is where the “emulsion breaker” (or “demulsifier”) comes in.Ĭhemical Emulsion Breakers Help Centrifuges Separate Even Tightly Bound Emulsions This results in a “tightly bound emulsion”-one that won’t separate on its own and may even prove challenging to separate with a centrifuge. Instead of drifting together and coalescing into bigger droplets, and eventually forming their own layer, the oil can remain dispersed in solution, free-floating throughout the water. Note that the “water-loving” heads point out, toward the water, while the “oil-loving” tails point in, protecting the oil droplet. The emulsifier gathers around the dispersed micro-droplets of oil (as shown on the right of the figure below). (In many cases, that tail isn’t just hydrophobic, but actually lipophilic-”oil-loving.”) That’s because these substances tend to be composed of molecules that have a hydrophilic (“water-loving”) head and a hydrophobic (“water-fearing”) tail. But the presence of emulsifiers, surfactants, co-surfactants, co-solvents, or other substances can complicate this.


 0 kommentar(er)
0 kommentar(er)
